Key Takeaways Mid-study RTSM migration is feasible and strategic for preserving trial integrity amid growing study complexity. Signs to change..


Key Takeaways Mid-study RTSM migration is feasible and strategic for preserving trial integrity amid growing study complexity. Signs to change..
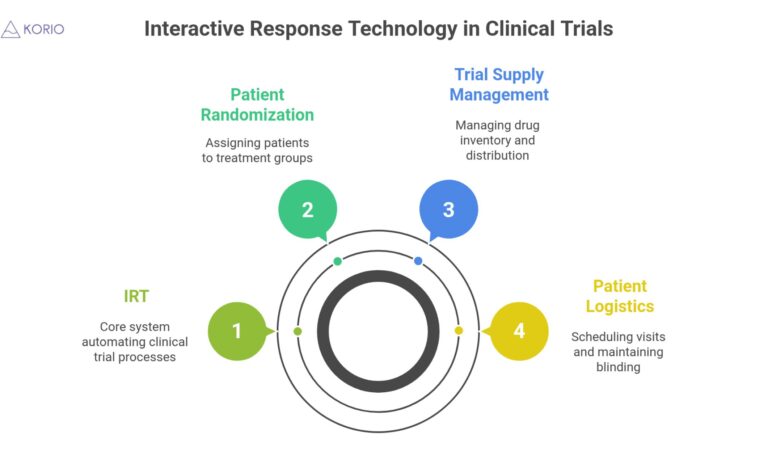
Key Takeaways IRT is a software system that automates patient randomization, drug supply, and visit scheduling in clinical trials. It..

In this recorded webinar presentation, join clinical trial supply management experts Ana-Zeralda Canals Hamann, Associate Director of Clinical Trial Supply..

What would you do if your Randomization and Trial Supply Management system (RTSM) couldn’t handle your protocol’s latest amendment, or..

When selecting a Randomization and Trial Supply Management (RTSM) system, are you balancing short-term efficiency with long-term success? Timeline efficiency..

DOYLESTOWN, Pa., April 8, 2025 — Korio, Inc., the pioneering Randomization & Trial Supply Management (RTSM) technology company, today announced..

Feeling Trapped by your Current RTSM Solution? How to exit your provider with minimal disruption. Doylestown, PA – March..

While many Sponsors rely on CROs for most clinical development functions, delegating RTSM vendor selection and management may create undesirable..

In a recent episode of RTSM Time, host Paul Hughes, Director of RTSM at Johnson & Johnson, sat down with..

Ever wonder why your “quick” RTSM change took 3 months? The truth is: these frustrating delays aren’t inevitable. They’re symptoms..